Find the total sum of valence electrons that each atom contributes to the molecule or polyatomic ion. A h b p.

Practice With Lewis Dot Diagrams Electron Dot Diagrams Chemistry Worksheets Graphing Quadratics Teaching Chemistry
Chemists uselewis dot structuresto show the valence electrons of an element as dots.
Valence electrons lewis dot structure worksheet. For those of you that enjoy such things some more lewis structures to draw. Valence electrons and dot structures. 8 c2h5oh ethanol 9 n2f4.
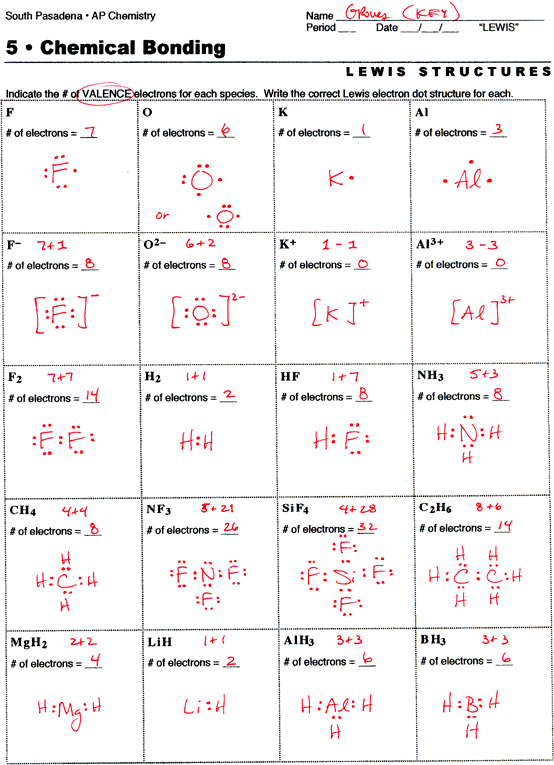
Draw the lewis structures for the following compounds. A lewis electron dot diagram or electron dot diagram or a lewis diagram or a lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Some of the worksheets for this concept are chemistry valence electrons lewis dot structures work name date chemistry valence electrons lewis dot lewis structures of atoms lewis dot structures and molecule geometries work electron dot lewis structures practice problems h s.
A h b p c ca d ar e cl f al. Determine the total number of valence electrons in the formula. Electrons are shown as dots around the chemical symbols of the atoms and bonds are represented as lines.
The number of dots equals the number of valence electrons in the atom. Since bonding involves the valance shell electrons only it is only necessary to illustrate those outer electrons. View assignment valence electrons and lewis dot structure worksheet answers from chem 101 at pascack hills high.
Yet more lewis structures. 10 sf6 lewis structures practice worksheet. Lewis structures on your notes try these elements on your own.
Lewis structures on your worksheet try these elements on your own. Since bonding involves the valence shell electrons only it is only necessary to illustrate those outer electrons. Lewis dot structures to show the valance electrons of an element as dots.
The dot formulas are sometimes referred to as lewis dot structures after the chemist who first introduced them in 1916 g n. Electron dot structure or lewis dot diagram gilbert lewis a notation showing the valence electrons surrounding the atomic symbol. Lewis structures practice worksheet 30 pps.
Valence electrons lewis dot structures. Lewis dot structures answer key displaying top 8 worksheets found for this concept. Displaying top 8 worksheets found for valence electrons and dot structures.
Lewis dot structures and molecule geometries worksheet answer key 1 lewis dot structures and molecule geometries worksheet answer key how to draw a lewis dot structure 1. Identify the number of valence electronsand draw the lewis dot structure notes. Some of the worksheets for this concept are lewis dot structures and molecule geometries work lewis electron dot structure answers lewis dot structure answer key lewis structures practice work work 14 work 13 d epa rtm ent of che m istry u niversity of t exa s at atomic protons neutrons electrons.

Lewis Dot Diagram Worksheet Chemistry Worksheets Science Worksheets Teaching Chemistry

Organic Chemistry High School Organic Chemistry School High School Fur Organische C In 2020 Chemistry Classroom Teaching Chemistry Chemistry Worksheets

Electron Dot Diagram Worksheet Worksheets For School Newpcairport Worksheets Chemistry Worksheets Word Problem Worksheets

Lewis Dot Structure Mini Lesson And Worksheet Chemistry Worksheets Mini Lessons Teaching Chemistry

Science Electronic Structure And The Periodic Table Teaching Chemistry Chemistry Classroom Chemistry Lessons

Printables Lewis Dot Structure Worksheet Lemonlilyfestival Chemistry Worksheets Ionic Bonding Electrons

A Truncated Version Of The Periodic Table Showing Lewis Dot Structures For The First 20 Elements Chemistry Lessons Teaching Chemistry Middle School Chemistry

Lewis Structures Valence Electron Diagrams For 1st 20 Elements Distance Learning Diagram Electron Configuration Compare And Contrast

Atomic Structure Worksheet Teaching Chemistry Chemistry Worksheets Chemistry Classroom

Lewis Structures Worksheet Video Worksheet With Answers Practices Worksheets Worksheets Persuasive Writing Prompts
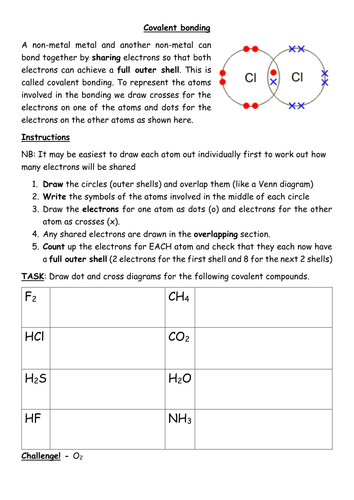
Lewis Dot Structure Covalent Bonds Worksheets Chemistry Worksheets Exam Study Chemistry

Covalent Bonding Worksheet Covalent Bonding Worksheet Covalent Bonding Ionic Bonding

20 Lewis Dot Diagram Worksheet In 2020 Teaching Chemistry Chemistry Classroom Chemistry Education

Lewis Dot Structures Cont Chemical Bond Chemistry Notes Chemistry Worksheets

Valence Electrons And The Periodic Table Worksheet Template Chemistry Education Printable Worksheets

Valence Electrons And Lewis Dot Structure Worksheet Answers Awesome Valence Electrons And Lewis Practices Worksheets Chemistry Worksheets Worksheet Template

Lewis Dot Worksheets Teaching Chemistry Chemistry Classroom Chemistry Worksheets

Lewis Dot Diagram Worksheet Chemistry Worksheets Chemistry Classroom Teaching Chemistry

Lewis Structure Practice Worksheet Unique Chemistry 162 Exam Study 2 Guide In 2020 Chemistry Worksheets Teaching Chemistry Chemistry