Material in this phase has volume and shape. T 12 54 kj 40 08 kj 6 15 kj 58 77 kj.

Specific Heat And Phase Change Ditto
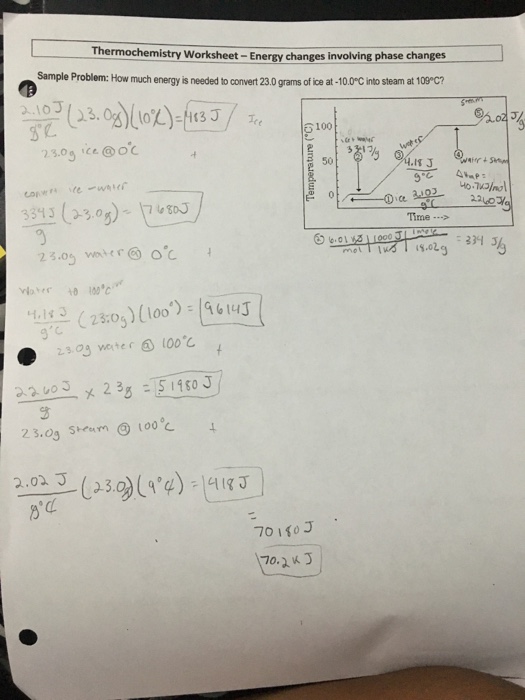
Calculations for temperature and phase change worksheet the heat of fusion of ice is 79 7 cal g.

Phase change calculations worksheet answer key. Q m h fus heat to boil. Ice 2 06 j g c water 4 184 j g c steam 2 03 j g c. The heat of vaporization of water is 540 cal g.
How much energy is required to melt 100 0 grams of ice. Phase change worksheet answer sheet 1 a 12 oz. How much energy is required to melt 100 0 grams of ice.
Some of the worksheets for this concept are calculations for temperature and phase change work heat with phase change work phase change simulation work phase change calculation work name date per thermochemistry work energy changes involving phase name per work heating curve of watercalculations. Heat to change the temperature. Phase change worksheet word bank.
How much energy is required to vaporize 234 5 g of water. Calculations for temperature and phase change worksheet the heat of fusion of ice is 79 7 cal g. 6 how many joules are required to heat 75 grams of water from 85 c to 185 c.
Now add the amount of heat q from each part of the answer. Displaying top 8 worksheets found for phase change calculation. How many joules are released when a can of soda is cooled from 25 degrees celsius room temperature to 4 degrees celsius the temperature of a refrigerator.
The heat of vaporization of water is 540 cal g. Q m t s heat to melt. 251 845 kj start with specific heat because the water is frozen and must heat up from 85 c to o c before it can go through a phase change.
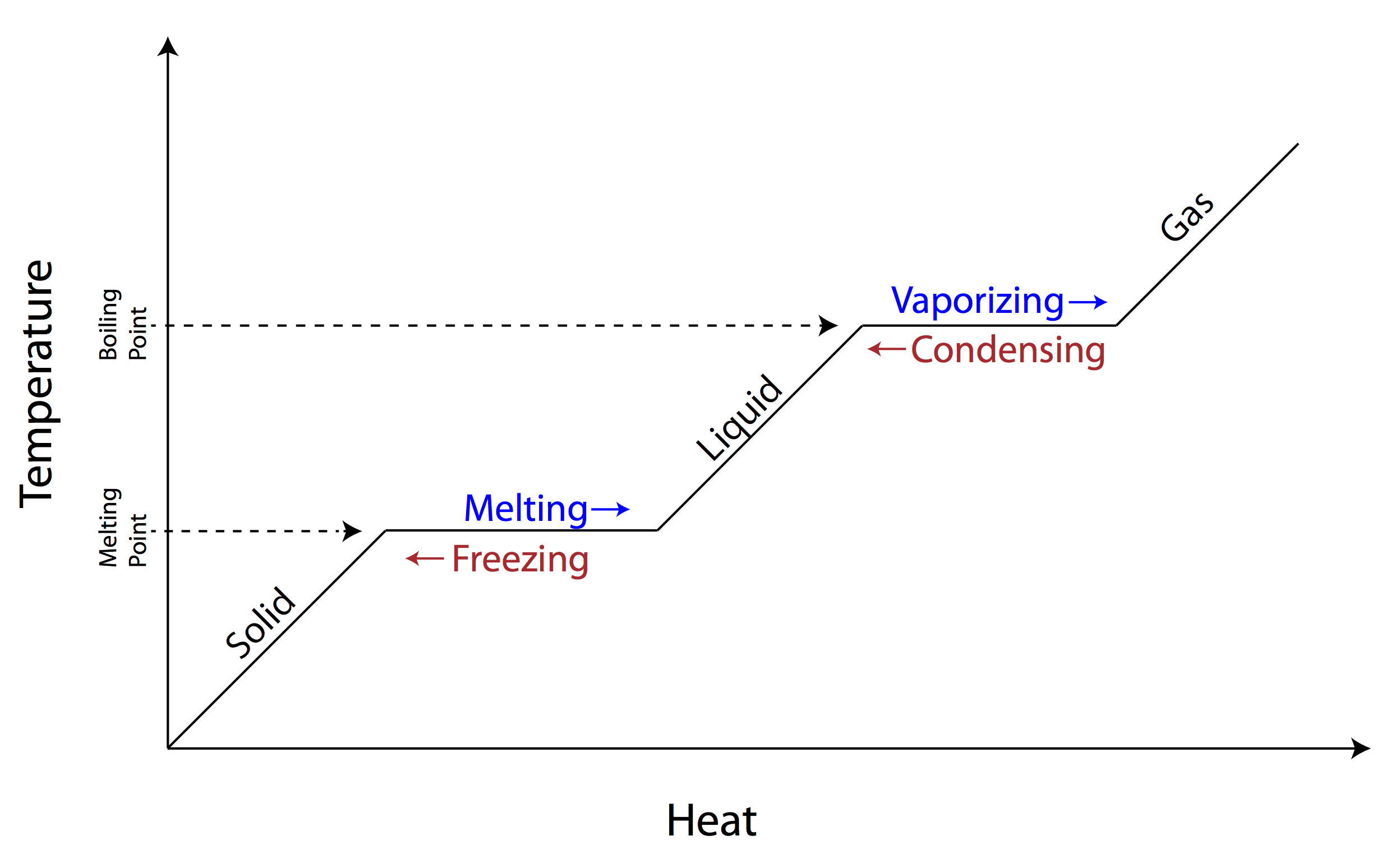
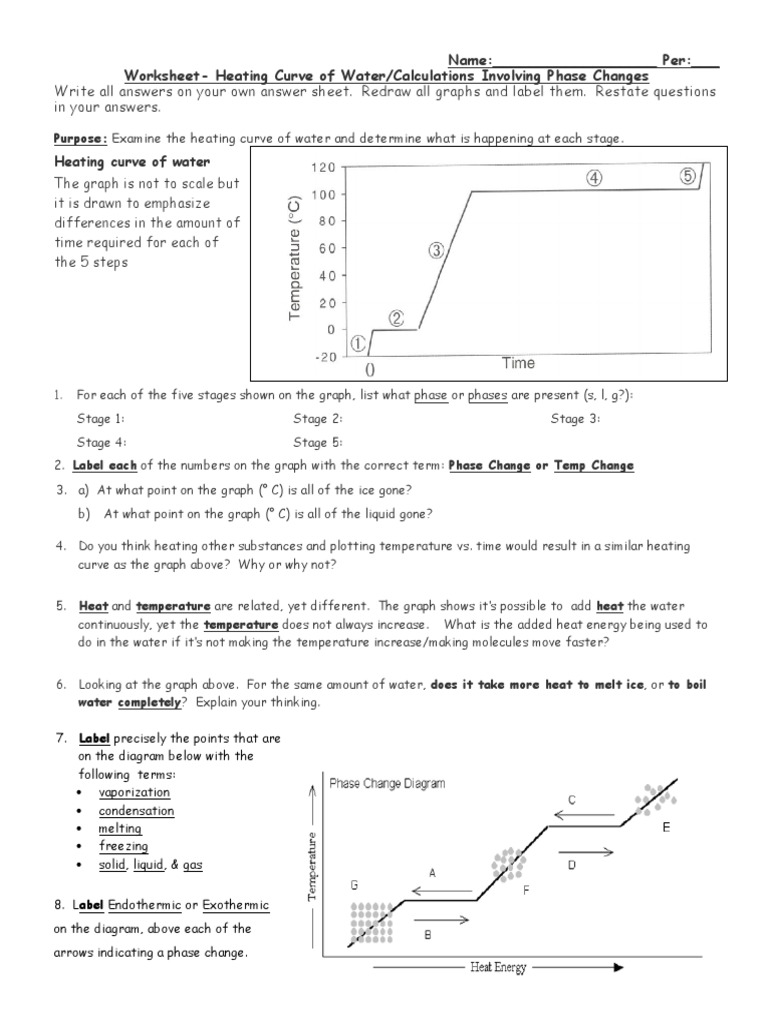
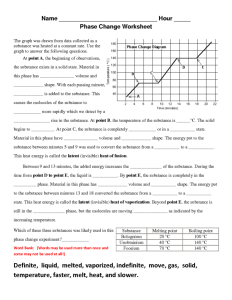
Report the answer using the correct number of significant figures. The graph was drawn from data collected as a substance was heated at a constant rate. Use the graph to answer the following questions.
Use the graph to answer the following questions. At point a the beginning of observations the substance exists in a solid state. The heat capacity of liquid water is 4 18 j gram x oc.
Can of soda weighs about 450 grams. Q m h vap for water. H fus 334 j g h vap 2260 j g specific heat.
How much energy is required to vaporize 234 5 g of water. Calculating energy for changes of phase to calculate heat. Report the answer using the correct of significant figures.
Some can be used more than once gas temperature infinite slower liquid melt vaporizing heat solid definite faster cool move energy the graph was drawn from data collected as a substance was heated at a constant rate.

Is Boiling Water Endothermic Or Exothermic Water Ionizer

Section 251 Nuclear Radiation Worksheet Answers Kids Activities

Worksheet Heat And Heat Calculations
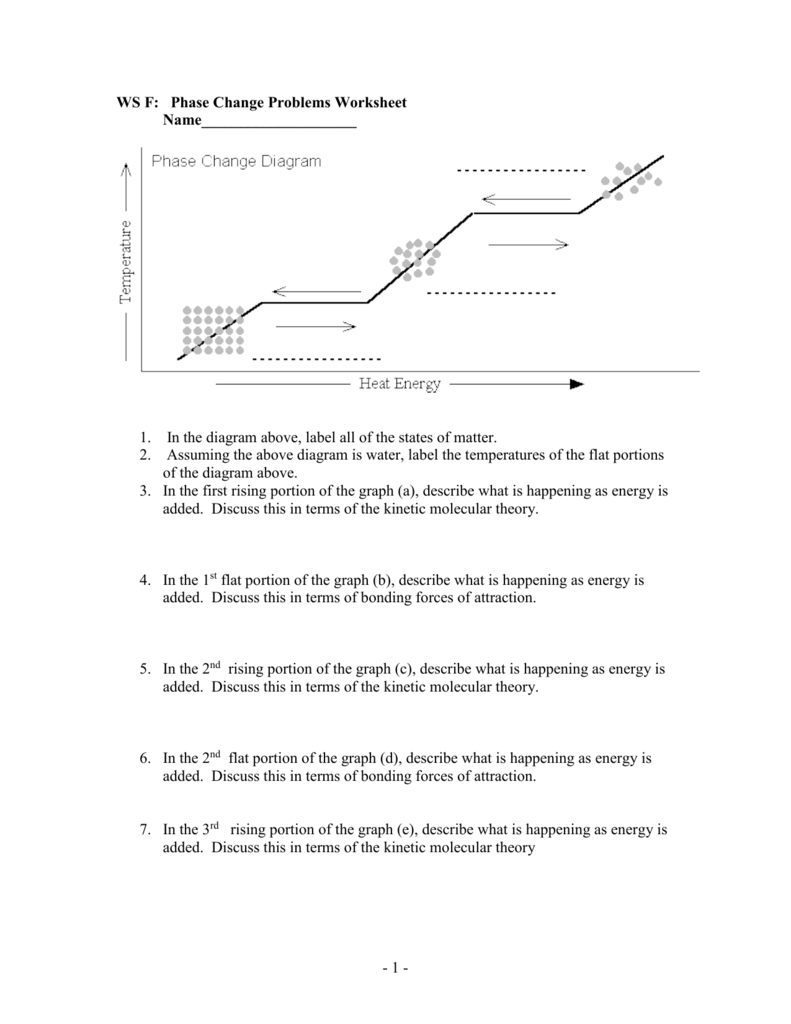
Ws F Phase Change Problems Worksheet
Https Www Studocu Com En Us Document University Of Houston Downtown Introductory Chemistry Tutorial Work Ws F Phase Change Problems Worksheet 7012135 View
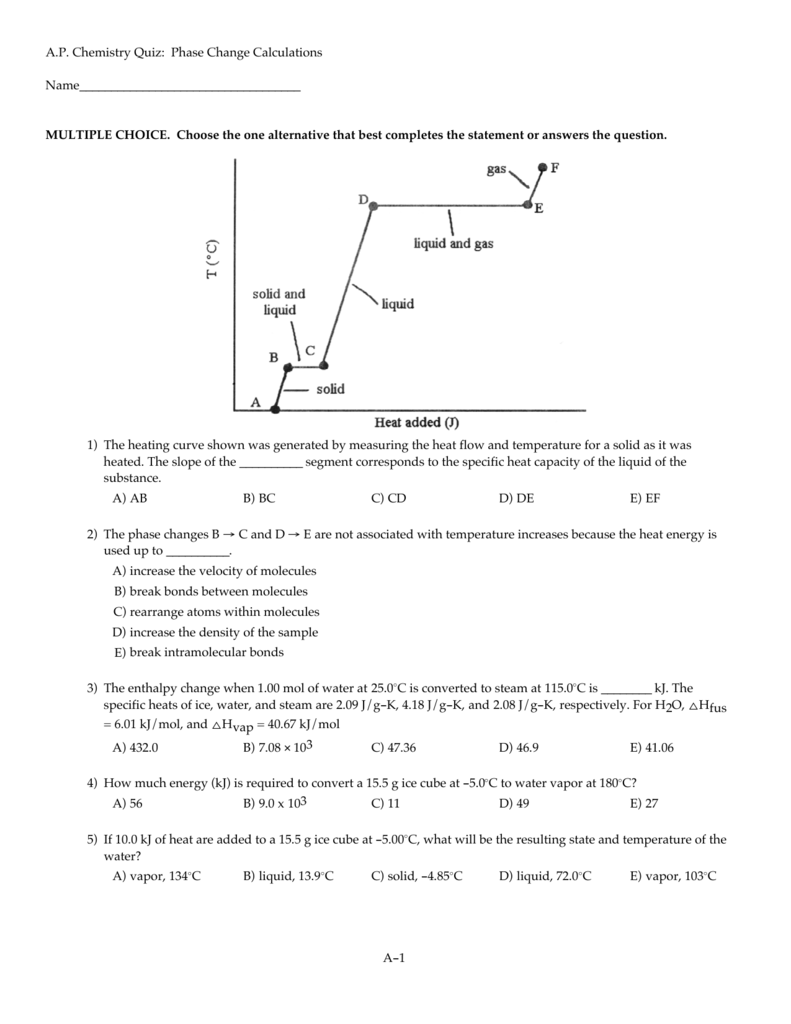
A P Chemistry Quiz Phase Change Calculations

Heat Transfer Lab Worksheet Answers Kids Activities
Heat Involving Phase Changes Heat Continuum Mechanics
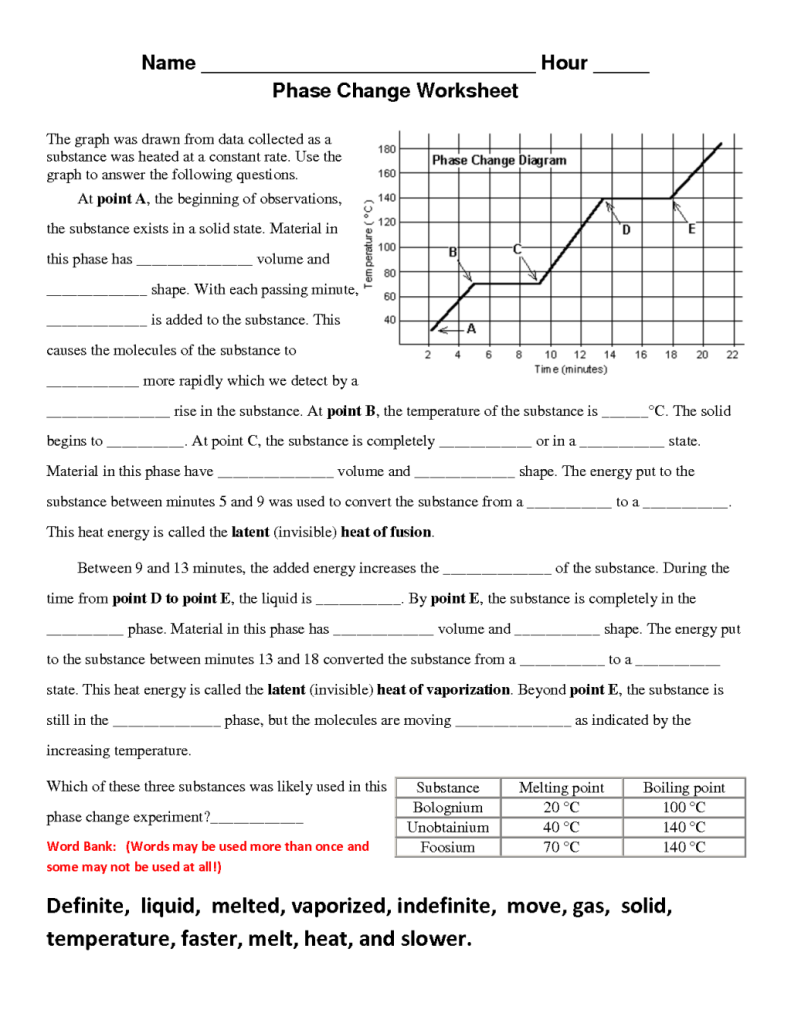
Phase Change Worksheet Answers Worksheets Graphing Algebra Graphs
Solved Thermochemistry Worksheet Energy Changes Involving Chegg Com

Solved Thermochemistry Worksheet Energy Changes Involvin Chegg Com
Thermochemistry Worksheet 1 Answers Promotiontablecovers

Honors Chemistry Heat And Change Of Phase Problems

Phase Change Calculation Worksheet Q Mcpaˆ T Q For A Phase
Heating Curve Of Water Worksheet Phase Matter Phase Transition